

What are the rules related to assigning oxidation numbers?

For instance, the sum of the value of oxidation numbers for SO 4 2- is -2.ġ. The sum of the value of oxidation numbers of all the present atoms in any neutral compound is 0.Īlso, the sum of the value oxidation numbers in any given polyatomic ion is always equal to the value of the charge of the ion. The oxidation number of Cl turns out to be -1 in HCl, but this number becomes +1 in HOCl. The oxidation number of elements present in Group VIIA in a compound is -1, except for the case when that element is conjoined with one having a higher value of electronegativity. The oxidation number of elements present in Group IIA in a compound is +2. The oxidation number of elements in Group IA in a compound is +1. Anomalies include OF2 because F turns out to be more electronegative than O, and BaO2, due to the construction of the peroxide ion, which can be expressed as (O-O) 2. The oxidation number of oxygen in the case of compounds is typically -2.
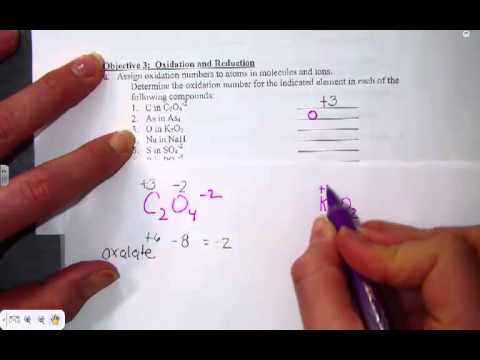
The oxidation number of hydrogen remains -1 in compounds, including elements that have less electronegativity than hydrogen, as in NaH. The expected oxidation number of hydrogen is plus 1. For instance, the oxidation number of Na 3- is 3. The oxidation number in the case of a monatomic ion is always equal to the value of the charge of the ion. The oxidation number of any free element always remains 0. The pattern is that the cation is always written first in a formula, which is then followed by the anion. Here are some of the rules for assigning oxidation numbers.
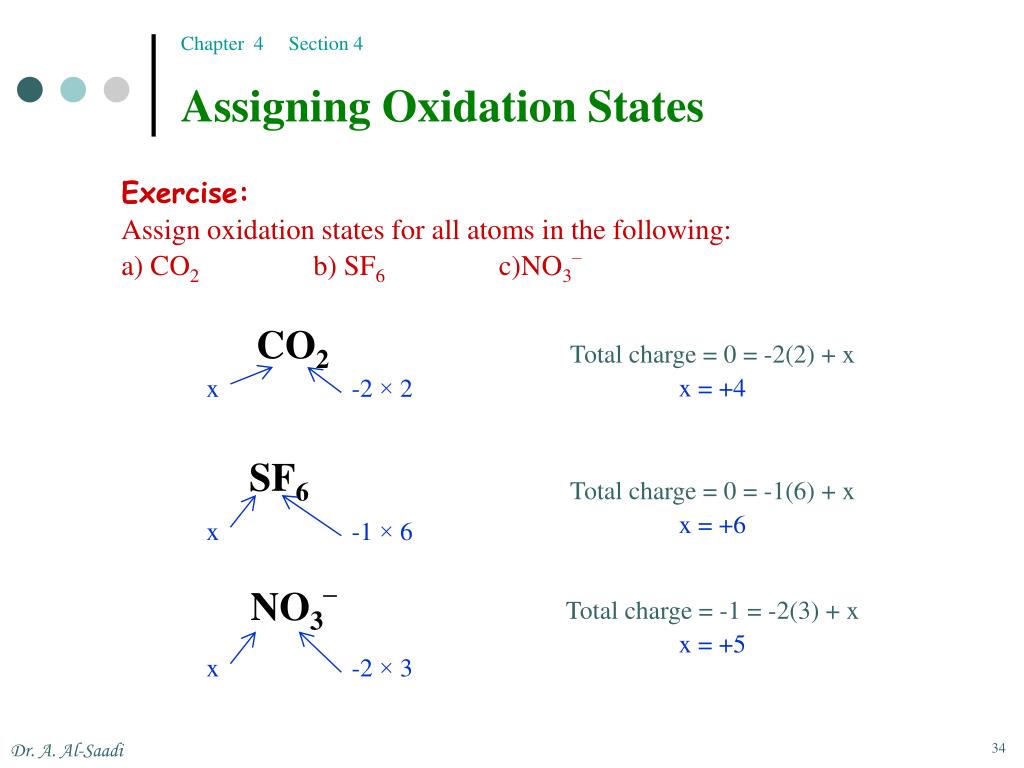
Source RULES FOR ASSIGNING OXIDATION NUMBERS Let’s learn about oxidation in this guide. Have you ever wondered why? It is because the metals get oxidized and develop rust when left untreated. Have you seen that there is a layer of rust forming on the surface of any of the metal rods around you? Most often metal surfaces are painted, or a coating is applied to decrease rusting.


 0 kommentar(er)
0 kommentar(er)
